7 years ago Why wouldn’t the 2,3,4 – trifluoropentane molecule have a larger boiling point than the nonane molecule? The TFP molecule has the electronegative fluorine which should create a dipole and hydrogen bond with other TFP molecules.
ALEKS – Predicting the Relative Boiling Points of Pure Substances – YouTube
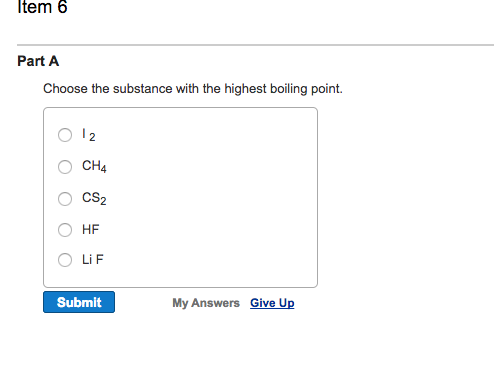
Chemistry Choose the substance with the highest boiling point. A F2 B CH4 C CO2 D CH3CH3 E CH3OH Choose the substance with the highest boiling point. A F2 B CH4 C CO2 D CH3CH3 E CH3OH Chemistry for Engineering Students 4th Edition ISBN: 9781337398909 Author: Lawrence S. Brown, Tom Holme Publisher: Lawrence S. Brown, Tom Holme

Source Image: dreamstime.com
Download Image
Another isomer of $\ceC10H22$, 2,4,6-trimethylheptane has boiling point of $\pu144.8 ^\mathrmoC$, which is also lower than that of n-nonane. All of these examples means, in order to predict boiling point simply by molecular weights, we may need additional information such as molecules structural features, etc.
Source Image: socratic.org
Download Image
Solved Choose the substance with the highest boiling point. | Chegg.com
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris) 2.11: Intermolecular Forces and Relative Boiling Points (bp) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The relative strength of the intermolecular forces (IMFs) can be used to predict the

Source Image: numerade.com
Download Image
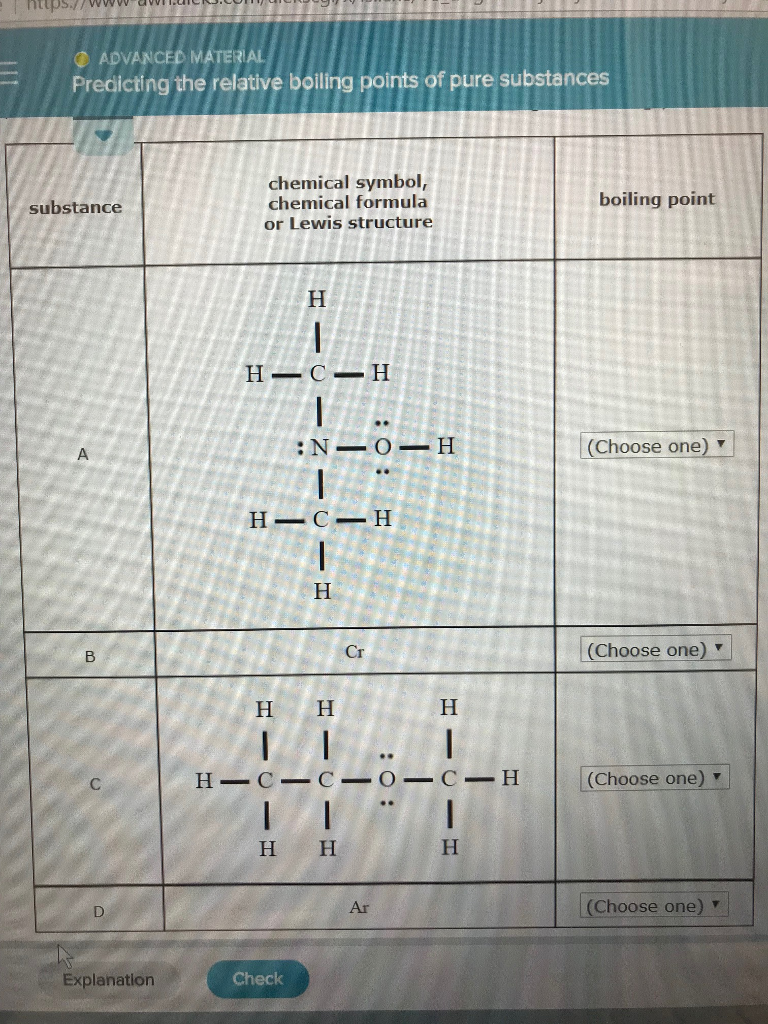
Choose The Substance With The Highest Boiling Point
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris) 2.11: Intermolecular Forces and Relative Boiling Points (bp) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The relative strength of the intermolecular forces (IMFs) can be used to predict the
For example, the boiling point of water is 100 °C. The boiling point depends on the pressure. Data given in this calculator refer to normal pressure, that is 1013,25 hPa. The relationship between boiling point and pressure is as follows: T b = [ 1 T 0 − R ln ( P b P 0) Δ H e v a] − 1.
SOLVED: 2 Look at the table below Substances A B; C and D all have covalent bonds. Substance Melting point (PC) Boiling point (‘C) 125 90 B 170 C 2200 3900 D
Jan 23, 2023First there is molecular size. Large molecules have more electrons and nuclei that create van der Waals attractive forces, so their compounds usually have higher boiling points than similar compounds made up of smaller molecules. It is very important to apply this rule only to like compounds.
Solved Rank the elements or compounds in the table below in | Chegg.com
Source Image: chegg.com
Download Image
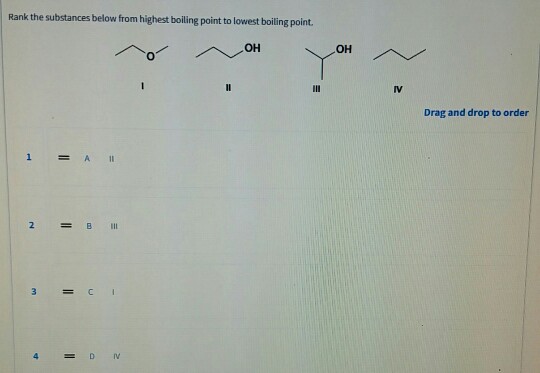
Solved Rank the substances below from highest boiling point | Chegg.com
Jan 23, 2023First there is molecular size. Large molecules have more electrons and nuclei that create van der Waals attractive forces, so their compounds usually have higher boiling points than similar compounds made up of smaller molecules. It is very important to apply this rule only to like compounds.
Source Image: chegg.com
Download Image
ALEKS – Predicting the Relative Boiling Points of Pure Substances – YouTube
7 years ago Why wouldn’t the 2,3,4 – trifluoropentane molecule have a larger boiling point than the nonane molecule? The TFP molecule has the electronegative fluorine which should create a dipole and hydrogen bond with other TFP molecules.

Source Image: youtube.com
Download Image
Solved Choose the substance with the highest boiling point. | Chegg.com
Another isomer of $\ceC10H22$, 2,4,6-trimethylheptane has boiling point of $\pu144.8 ^\mathrmoC$, which is also lower than that of n-nonane. All of these examples means, in order to predict boiling point simply by molecular weights, we may need additional information such as molecules structural features, etc.
Source Image: chegg.com
Download Image
Solved 6. Look up the boiling points of the following | Chegg.com
Jan 18, 2024You can also use our boiling point calculator instead and save yourself some time. This calculator has the values of P₁ and T₁ set to 1013.25 hPa and 100 °C, respectively. These values correspond to the normal atmospheric pressure at the sea level and boiling point of water. You need to set different values if you are calculating the
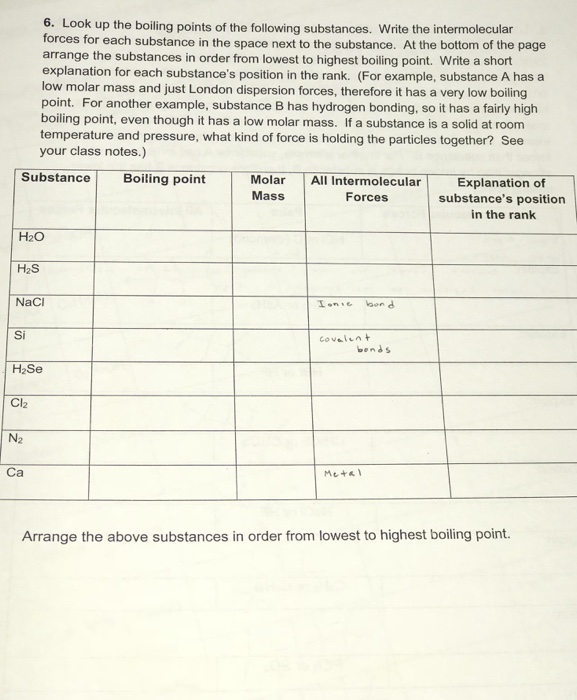
Source Image: chegg.com
Download Image
SOLVED: Rank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris) 2.11: Intermolecular Forces and Relative Boiling Points (bp) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The relative strength of the intermolecular forces (IMFs) can be used to predict the
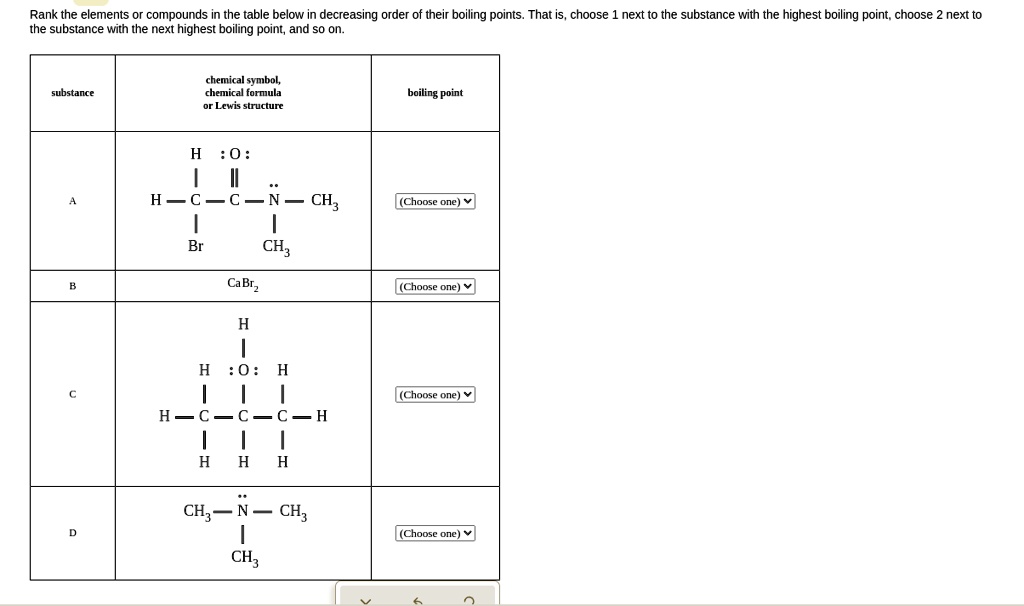
Source Image: numerade.com
Download Image
Computational Insights into the Selecting Mechanism of α-Amylase Immobilized on Cellulose Nanocrystals: Unveiling the Potential of α-Amylases Immobilized for Efficient Poultry Feed Hydrolysis | Bioconjugate Chemistry
For example, the boiling point of water is 100 °C. The boiling point depends on the pressure. Data given in this calculator refer to normal pressure, that is 1013,25 hPa. The relationship between boiling point and pressure is as follows: T b = [ 1 T 0 − R ln ( P b P 0) Δ H e v a] − 1.

Source Image: pubs.acs.org
Download Image
Solved Rank the substances below from highest boiling point | Chegg.com
Computational Insights into the Selecting Mechanism of α-Amylase Immobilized on Cellulose Nanocrystals: Unveiling the Potential of α-Amylases Immobilized for Efficient Poultry Feed Hydrolysis | Bioconjugate Chemistry
Chemistry Choose the substance with the highest boiling point. A F2 B CH4 C CO2 D CH3CH3 E CH3OH Choose the substance with the highest boiling point. A F2 B CH4 C CO2 D CH3CH3 E CH3OH Chemistry for Engineering Students 4th Edition ISBN: 9781337398909 Author: Lawrence S. Brown, Tom Holme Publisher: Lawrence S. Brown, Tom Holme
Solved Choose the substance with the highest boiling point. | Chegg.com SOLVED: Rank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2
Jan 18, 2024You can also use our boiling point calculator instead and save yourself some time. This calculator has the values of P₁ and T₁ set to 1013.25 hPa and 100 °C, respectively. These values correspond to the normal atmospheric pressure at the sea level and boiling point of water. You need to set different values if you are calculating the